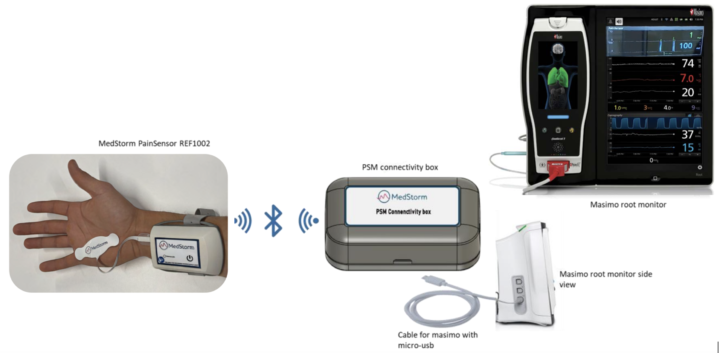
QA-log
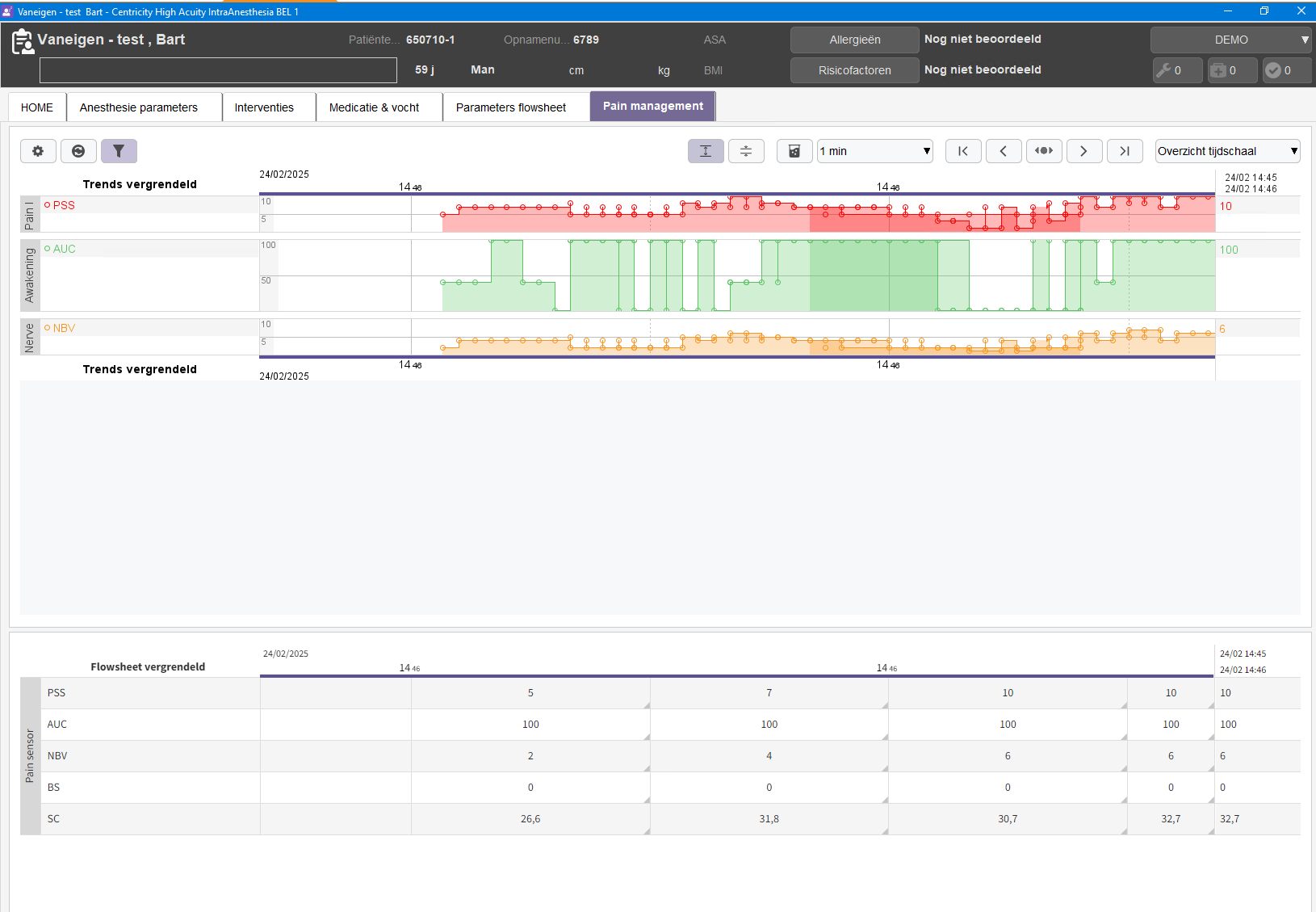
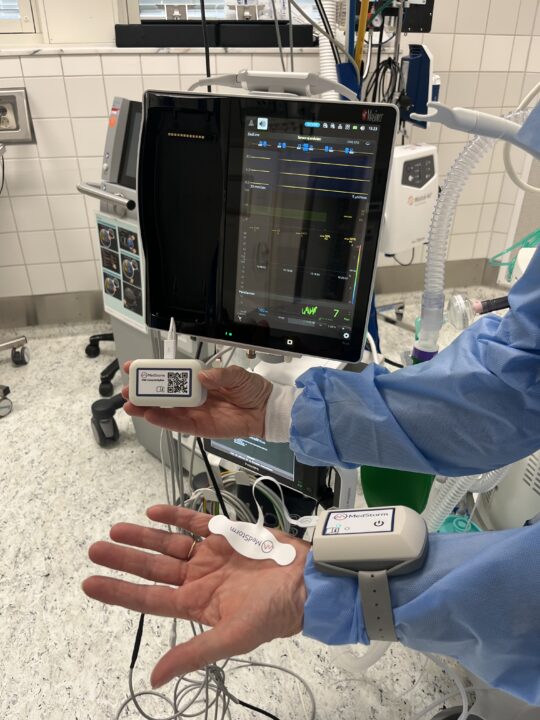
QA-log is a new software feature from MedStorm that allows safe and easy transfer of data from the patient to your EMR system (e.g. Metavision), with the press of just one button:
The QA-log button gives you the option to add input and outcome parameters, which are flexible to define from the user. The application will improve quality when implementing new patient treatment, as well as help with the monitoring of existing treatments. For research purposes, this will help with the collecting and sampling of patient data, and considerably reduce the work load of the researcher.
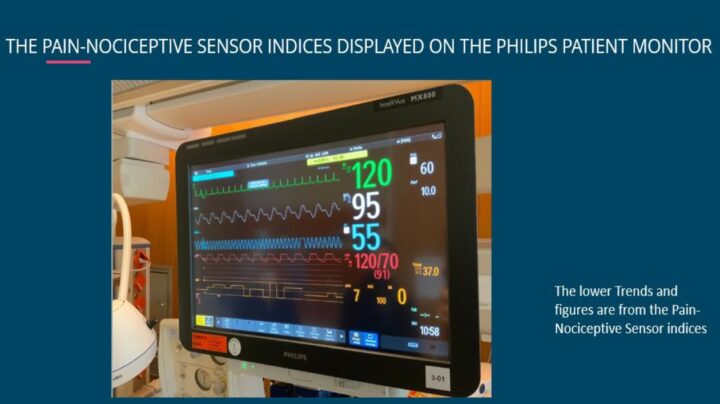
To secure the safety of the patient, the QA-log uses HL7, which is the highest standard for safely transferring patient data to electronic documentation sites. By using MedStorm´s QA-log button you can therefore safely and continuously transfer your own defined inputs to your electronic documentation site, for the best possible treatment of your patient and the easiest work for you.