MedStorm Innovation
MedStorm Innovation is a medical technology company pioneering in developing a method based on analysing skin sympathetic nerve activity with acetyl-choline acting on muscarinic receptors mirrored by changes in skin conductance or emotional sweating to assess pain and nociceptive stimuli in all ages.
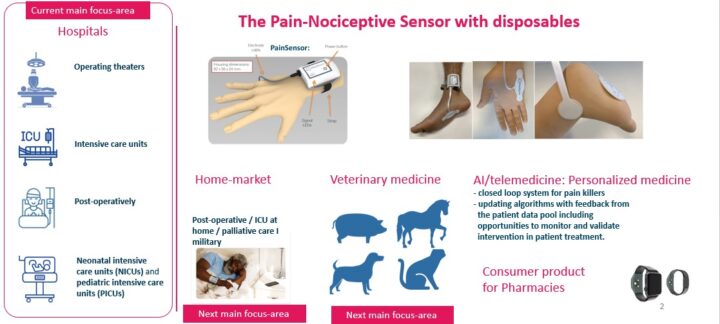
The founder and CEO of Med-Storm Innovation, Prof. Hanne Storm MD.PhD, University of Oslo, Norway, started examining the opportunity to use the skin conductance technology to assess pain in infants at the NICU, Rikshospitalet, Oslo University hospital, Oslo, already in 1999. In 2009 one CE-certified research tool, the PainMonitor, to assess skin conductance was developed to assess pain and nociceptive stimuli for the NICUs, PICUs, postoperatively and in the operating theatres. There were customers worldwide purchasing the PainMonitor publishing independent studies showing that the PainMonitor can be used to titrate the correct dose of analgesia / anti-nociceptive drugs. After request from the academic customers, Med-Storm Innovation developed a minaturized and wearable Pain-Nociceptive Sensor for clinical purposes which obtained MDR-CE-certification in 2023. Then also indices to assess regional nerveblock and withdrawal sympotoms were finalized. All the indices are in the Pain-Nociceptive Sensor devices. More than 80 validation studies and 5 theses are published through peer-reviewed journals. There are 12 approved patents on the algorithms for the indices and the proprietary sensors. Through worldwide sales and grants from the European Comittee and the Norwegian governments, Med-Storm Innovation has been able to build sensitive and specific skin conductance device, the Pain-Nociceptive Sensor. The Pain-Nociceptive Sensor is connected to the Philips patient monitors, Masimo ROOT, Mindray’s patient monitor when using their iPC, and the Draeger patient monitors when using SDC, as well as electronic medical records when using HL7. Recently Med-Storm Innovation developed an application to secure quality of the patient treatment where the patient data are stored locally or trasferred to the electronic medical records by HL7.
The Pain-Nociceptive Sensor is a low cost, wireless and wearable device with proprietary electrodes. Our accurate, real-time, and objective pain and nociceptive assessment tool offers a simple, real-time readout number to improve pain management and save costs for the hospitals.